16. Clinical Guidelines for Pain Management in Intratumoral Chlorine Dioxide Injection Therapy
Preface
Intratumoral chlorine dioxide (ClO₂) injection therapy (referred to as "ClO₂ therapy" hereafter) is an innovative, minimally invasive anti-tumor treatment method based on localized action. By directly injecting an appropriate dose of chlorine dioxide into tumor tissues, ClO₂ therapy can specifically destroy cancer cells while minimizing damage to surrounding healthy tissues, effectively reducing the risk of adverse reactions for patients.
Due to its minimally invasive nature, this therapy demonstrates significant potential for development in the field of anti-tumor treatments. To standardize pain management measures during the clinical application of this therapy, ensure patient safety, and optimize treatment outcomes, a team of domestic experts has combined practical experience with the latest research findings to develop this "Clinical Guidelines for Pain Management in Intratumoral Chlorine Dioxide Injection Therapy." The purpose of these guidelines is to provide comprehensive anesthesia and pain management protocols, guide healthcare professionals in managing potential adverse reactions during treatment, and serve as a scientific foundation for advancing the clinical application of ClO₂ therapy.
Pain management is a crucial component of ClO₂ therapy, playing a vital role in ensuring smooth treatment implementation and a positive patient experience. These guidelines focus on anesthesia and pain management strategies during the procedure and perioperative period, including local anesthesia, regional nerve blockade, spinal anesthesia, and general anesthesia. They provide detailed recommendations on medication selection, treatment procedures, and the management of potential complications, making them a valuable reference for healthcare providers at all levels.
We hope that the promotion and implementation of these guidelines will further enhance the safety and standardization of ClO₂ therapy, bring greater benefits to patients, and drive progress and advancement in the field of anti-tumor therapies.
Part 1 | Preparation of Medications and Equipment
(1) Medication Preparation
Pain Management Medications:
Local Anesthetics: Lidocaine, bupivacaine, ropivacaine, primarily for local anesthesia along the puncture path and pain control in surrounding tumor tissues.
Analgesics: Includes opioids (e.g., fentanyl, sufentanil, hydromorphone, morphine) and nonsteroidal anti-inflammatory drugs (NSAIDs, e.g., parecoxib sodium, flurbiprofen axetil) or acetaminophen, which can be combined to alleviate burning pain or discomfort caused by the injection.
Sedatives: Continuous infusion of midazolam, dexmedetomidine, or remimazolam to reduce patient tension and anxiety.
ClO₂ Therapy-Specific Medications:
Antioxidants: Vitamin C (antioxidant) to address potential oxidative reactions or suspected tissue damage caused by chlorine dioxide diffusion.
Buffer Solutions: Saline for diluting or neutralizing chlorine dioxide.
Emergency Medications:
Common Medications: Atropine (for reflex bradycardia), isoproterenol, epinephrine, dopamine.
Antiallergic Drugs: Corticosteroids and antihistamines (e.g., dexamethasone, hydrocortisone, diphenhydramine) to prevent or manage allergic reactions.
Symptomatic Medications: Volume expanders like mannitol, benzodiazepines for sedation, and antiemetics (e.g., ondansetron) for emergency use.
Other Medications:
Antiemetics, medications for reactive hypotension, and plasma expanders as needed.
(2) Preparation of Anesthesia and Auxiliary Equipment
Anesthesia Equipment:
Ventilators or anesthesia machines with intermittent positive pressure ventilation, suitable for patients requiring general anesthesia or airway protection.
Cardiopulmonary Resuscitation Equipment and Emergency Kits:
Includes intubation tools (e.g., indirect laryngoscopes/video laryngoscopes, endotracheal tubes, laryngeal masks), manual resuscitators, and CPR tools. Ensure appropriate sizes and tools are available for patients with unique body types or conditions.
Defibrillator:
A defibrillator must be available 24/7 to address potential parasympathetic reflex-induced cardiac arrest caused by local injection diffusion.
Continuous Oxygen Supply Systems:
Provide oxygen through centralized pipelines or oxygen cylinders. Ensure routine maintenance and usage records are up to date.
Multifunctional Monitoring Devices:
Capable of monitoring ECG, oxygen saturation, automatic respiration, and blood pressure. If necessary, monitor end-tidal carbon dioxide (ETCO₂). For MRI-guided procedures, use MRI-compatible monitors.
Suction Devices:
Equip negative pressure suction systems to prevent aspiration in patients who have not fasted or are prone to vomiting. These systems also help reduce respiratory secretions caused by allergic reactions to the injection.
Other Auxiliary Equipment:
Communication devices for rapid contact with the anesthesiology department and emergency center, resuscitation carts, and patient transport equipment.
Part 2 | Multimodal Pain Management
Multimodal pain management combines analgesics and methods with different mechanisms of action to address the various types of pain caused by intratumoral injections (e.g., puncture pain, chemical burn pain, and inflammatory reactive pain from diffusion).
1. Pain Management Methods
Pain from intratumoral chlorine dioxide injection is generally mild due to the minimally invasive nature of the procedure. Most pain originates from the puncture and chemical irritation of the injected solution, as well as stress responses in surrounding tissues. Pain can be managed with a single anesthetic or through the following multimodal combinations:
Local Anesthetics + NSAIDs/Acetaminophen Combination:
Recommended use of local anesthetics (e.g., 1%-2% lidocaine) with NSAIDs (e.g., parecoxib sodium).
Opioid Analgesics + Intravenous Sedatives Combination:
For patients with low pain tolerance, add opioids such as fentanyl or hydromorphone, combined with sedatives like dexmedetomidine or midazolam to alleviate anxiety.
2. Principles of Pain Management Combinations
Select single or combined pain management strategies based on the patient’s condition.
Prioritize drug combinations with fewer side effects to minimize drug accumulation and expedite recovery.
3. Recommended Pain Management Protocols
Protocol 1:
Local Anesthesia: Lidocaine (1%-2%) or ropivacaine (0.2%).
Adjunct Analgesia: NSAIDs (parecoxib sodium or flurbiprofen axetil).
Protocol 2:
Analgesics: Hydromorphone, sufentanil, or morphine (dose adjusted to patient needs).
Sedatives: Dexmedetomidine to maintain a calm but conscious state.
Protocol 3:
Combined Analgesia: Hydromorphone + flurbiprofen axetil.
Sedatives: Midazolam, with careful dose adjustment to avoid respiratory depression.
Protocol 4:
NSAIDs (e.g., parecoxib sodium).
Sedatives: Dexmedetomidine to reduce patient tension.
4. Recommended Use: Patient-Controlled Analgesia (PCA)
PCA is the preferred pain management strategy during the perioperative period, especially for patients requiring multiple intratumoral injections.
Advantages of PCA:
Rapid and effective pain relief with no significant blind spots.
Stable drug plasma concentrations.
Personalized control enhances patient comfort and satisfaction.
5. Recommended PCA Medications
Opioids: E.g., hydromorphone, sufentanil.
NSAIDs: E.g., parecoxib sodium, flurbiprofen axetil.
Sedatives: E.g., midazolam, dexmedetomidine.
Dosages should be tailored individually based on the patient’s condition, injection requirements, and therapeutic efficacy.
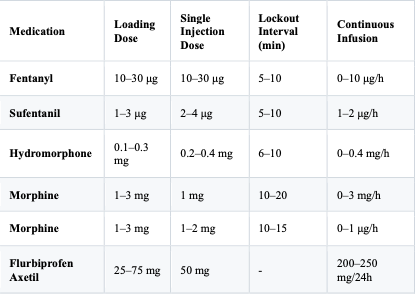
Table 1: Recommended Protocols for Common Patient-Controlled Analgesia (PCA) Medications
Note:
During combination therapy, patient respiratory function must be closely monitored.
“-” indicates that the lockout interval is not specified.
Part 3 | Combined Pain Relief and Sedation Protocols with Local Anesthesia, Regional Nerve Blocks, or Spinal Anesthesia
Local anesthesia, regional nerve blocks, or spinal anesthesia combined with pain relief and sedation are suitable for cases of intratumoral chlorine dioxide injection therapy when the surgical site is superficial, the procedure is relatively simple, and the surgery duration is short. These methods effectively alleviate pain during the injection process and enhance patient tolerance to treatment.
1. Local Anesthesia
Drug Selection:
It is recommended to use 1%-2% lidocaine or 0.2% ropivacaine for local infiltration anesthesia. This covers the injection path and the tumor’s surrounding tissue, reducing pain from puncture and injection.Application Scope:
Suitable for intratumoral injections in superficial or small-sized tumors.
2. Regional Nerve Blocks
Regional nerve blocks are administered by anesthesiologists based on the tumor location, targeting the pain conduction pathways of the tumor and its surrounding tissue. This method is particularly appropriate for treating deep or larger tumors.
Drug Selection:
Typically, 0.2%-0.5% ropivacaine or bupivacaine injection solutions are used.Drug Dosage:
Dosages should be adjusted according to the nerve innervation area and tumor size. Avoid overdosing to prevent motor nerve blockages.
Common Nerve Block Techniques:
Thyroid Tumors:
Use 0.2% ropivacaine for superficial cervical block, covering the superficial nerve distribution around the thyroid gland.Lung Tumors:
Employ 0.2%-0.5% ropivacaine for paravertebral nerve plexus block, serratus anterior plane block, or intercostal nerve block.Liver Tumors and Other Deep Tumors:
Choose appropriate nerve block techniques based on the tumor location, such as intercostal nerve block or transversus abdominis plane block.
3. Spinal Anesthesia
Spinal anesthesia is suitable for deep or larger tumors in intratumoral injection therapy, especially for tumors in the lower abdomen or pelvic region. The anesthesiologist selects the puncture site based on the tumor location to provide broader coverage and better analgesic effects.
Anesthesia Methods:
i. Epidural anesthesia only.
ii. Combined epidural and intrathecal block.
iii. Intrathecal block only.Characteristics:
i. Spinal anesthesia effectively blocks pain conduction and significantly reduces pain during treatment.
ii. It can be used for continuous postoperative analgesia.
iii. Drawbacks include longer procedure times and potential side effects such as hypotension and bradycardia, which must be managed promptly.Drug Selection:
i. Epidural Anesthesia: 1.5% lidocaine, 0.5% ropivacaine, or bupivacaine.
ii. Intrathecal Block: 0.5% ropivacaine or bupivacaine injection, with a single dose generally not exceeding 3 mL.Application Scope:
i. Procedures below the mid-thoracic plane: Epidural anesthesia or combined epidural and intrathecal block is recommended.
ii. Lower abdomen and pelvic tumors: Single-shot intrathecal block is preferable, as it acts quickly and offers excellent pain relief.
Part 4 | Pain Relief and Sedation Protocols for Intratumoral Chlorine Dioxide Injection Therapy
During intratumoral chlorine dioxide injection therapy, pain may arise from the chemical stimulation of the injected liquid, the increased pressure within the tumor tissue, and the inflammatory response of surrounding tissues. The primary objectives of pain relief and sedation are:
To alleviate injection pain, helping patients tolerate the puncture and injection process.
To reduce anxiety and tension, improving the treatment experience with sedative medications.
To avoid excessive pain by promptly identifying and managing significant discomfort during the injection process.
(1) Pain Relief and Sedation Protocol
Drawing from the ABCDE administration protocol used in high-intensity focused ultrasound (HIFU) therapy, the following stepwise protocol is recommended:
A: Administer fentanyl at 1 μg/kg (or an equivalent opioid).
B: Administer midazolam at 0.010-0.035 mg/kg (or an equivalent sedative).
C: Repeat Step A.
D: Administer half the dose of Step B.
E: Add 1-2 mg of midazolam as needed.
Notes:
After completing the ABCDE steps, any additional doses should be set at 1/4 of the initial effective loading dose.
The total midazolam dose should not exceed 0.15 mg/kg.
Adjust dosages and drug combinations flexibly according to patient tolerance.
Drug Alternatives:
Fentanyl: May be substituted with sufentanil, remifentanil, alfentanil, hydromorphone, oxycodone, or nalbuphine in equivalent doses.
Midazolam: May be substituted with remimazolam or dexmedetomidine.
(2) Standard for Effective Drug Loading Doses
The sedation depth should achieve a Ramsay sedation score of 3-4: the patient feels relaxed, appears drowsy but remains calm, and responds to gentle verbal calling or light tactile stimulation.
(3) Standards for Drug Addition
Additional drugs from Steps B, C, D, and E should be administered if, after Step A, any of the following occurs during treatment:
Agitation: The patient is unable to tolerate the treatment position.
Significant Pain: The injection area exhibits severe pain that cannot be alleviated through adjustment of the injection speed or dose.
(4) Standards for Treatment Termination
Treatment should be halted immediately under the following circumstances:
Radiating Limb Pain: May indicate nerve injury.
Unbearable Burning Sensation: In the skin of the injection area.
Unrelieved Pain: Pain remains intolerable even after completing Step E and adjusting treatment parameters.
(5) Monitoring Indicators and Management of Complications
Monitoring: Continuously monitor heart rate, respiration, blood pressure, and oxygen saturation during treatment.
Observation: Focus on changes in patient pain levels, vomiting, consciousness, and pupil reactions.
Management Principles: Adverse reactions to pain relief and sedation drugs should be managed according to general anesthesia protocols. Symptomatic supportive care should be administered if necessary.
Part 5 | General Anesthesia
General anesthesia is recommended for the following scenarios during intratumoral chlorine dioxide injection therapy:
Patients unable to cooperate with treatment:
Examples include children, individuals with severe anxiety, or those unable to tolerate the treatment position.Prolonged procedures:
Cases involving large tumor volumes, multiple injections, or complex injection processes.Lesions near sensitive areas:
For example, the chest wall, abdominal wall, bones, or regions with dense nerve distributions that may cause severe pain.
General anesthesia can be divided into intravenous-only anesthesia and intravenous-inhalational combined anesthesia. However, because intratumoral injection therapy is typically conducted outside operating rooms and lacks waste gas evacuation systems, intravenous-inhalational combined anesthesia is not recommended.
1. Intravenous Anesthesia
1.1 Intravenous Anesthesia Preserving Spontaneous Respiration
This method is suitable for most patients undergoing intratumoral injection therapy. It meets the requirements for pain relief and sedation and offers the following advantages:
Relatively simple operation with minimal equipment requirements.
Rapid induction and controllable depth of anesthesia.
Quick postoperative recovery with no intraoperative awareness.
1.2 Intravenous Anesthesia Without Spontaneous Respiration
This approach is ideal for patients requiring reduced diaphragm movement or controlled breathing, such as those undergoing lung tumor injections. These patients require the establishment of an artificial airway and the use of mechanical ventilation.
2. Personnel Requirements
For anesthesia performed in non-operating room environments, each treatment unit must be staffed with:
Anesthesiologist: At least one anesthesiologist with an attending physician qualification or higher.
Anesthesia Nurse: One dedicated anesthesia nurse per treatment unit to assist with anesthesia procedures and intraoperative management.
3. Patient Condition and Anesthesia Assessment
Before the procedure, the anesthesiologist should conduct a routine preoperative visit and assessment, collaborating with the surgeon to develop the anesthesia plan. The evaluation should include:
Newly developed conditions:
Such as acute upper respiratory infections or chest pain, which may affect anesthesia safety.Chronic conditions:
Such as hypertension or diabetes, requiring adjustments to preoperative and intraoperative medications based on the patient’s condition.Tumor-specific assessment:
Evaluate tumor size, location, and potential complications to formulate appropriate contingency plans.
4. Patient Preparation
Preoperative Fasting and Fluid Restriction:
No solid food for 6–8 hours before the procedure.
No clear liquids for 2 hours before the procedure (total intake not exceeding 400 mL).
Other Preparations:
Conduct respiratory training if necessary.
Obtain informed consent, ensuring that the patient and family understand the risks of anesthesia.
5. Selection of Anesthetic Drugs
General anesthetic drugs should be chosen for their rapid onset, ease of titration, and fast metabolic clearance. For patients with malignant tumors, who often have poor physical condition and multiple comorbidities, drug selection should be particularly cautious.
Recommended Drugs:
Sedatives: Midazolam, remimazolam, dexmedetomidine.
Intravenous Anesthetic Agents: Propofol, etomidate.
Analgesics: Sufentanil, remifentanil, alfentanil, hydromorphone, nalbuphine.
6. Selection of Artificial Airways
Artificial airways are used to ensure airway patency during anesthesia. Common options include:
Oropharyngeal airway.
Nasopharyngeal airway.
Laryngeal mask airway (LMA).
Endotracheal tube (if necessary).
The laryngeal mask airway is generally recommended as the first choice for artificial airway management due to its simplicity and reduced risk of airway injury. For lung tumor injection therapy or cases requiring reduced diaphragm movement, a bronchial blocker may be used.
7. Induction and Maintenance of General Anesthesia
7.1 General Anesthesia Induction
Based on the preoperative assessment, select fast-acting and short-duration drugs for induction. The following are recommended induction protocols:
Protocol 1:
Sedation: Midazolam 0.15–0.20 mg/kg (or remimazolam).
Induction: Propofol 1.0–2.0 mg/kg.
Analgesia: Combine with low-dose opioids (e.g., sufentanil 0.2–0.5 μg/kg, remifentanil 0.5–1.0 μg/kg).
Protocol 2:
Sedation: Midazolam 0.15–0.20 mg/kg.
Induction: Etomidate 0.3 mg/kg.
Analgesia: Combine with low-dose opioids (e.g., nalbuphine 0.2 mg/kg).
Protocol 3:
Sedation: Dexmedetomidine 0.5–1.0 μg/kg.
Induction: Propofol 1.0–2.0 mg/kg.
Analgesia: Combine with low-dose opioids (e.g., alfentanil 25–75 μg/kg).
Precautions:
The synergistic effects of sedatives and opioids may cause severe respiratory depression. Adjust dosages according to the patient’s condition.
Closely monitor the patient’s vital signs during induction to ensure safety.
7.2 General Anesthesia Maintenance
Continuously infuse propofol (2–6 mg/kg/h) combined with opioids.
Adjust drug dosages in real time based on intraoperative monitoring to maintain appropriate anesthesia depth.
8. Intraoperative and Recovery Monitoring and Management
8.1 Intraoperative Monitoring
Continuously monitor electrocardiogram, blood pressure, oxygen saturation, and end-tidal carbon dioxide levels.
Observe the patient’s anesthesia depth, pain responses, and intraoperative complications, and provide timely symptomatic treatment.
8.2 Recovery Phase Monitoring
Monitor the patient’s consciousness, airway patency, respiratory rate, and ventilation volume.
Record pulse oximetry, blood pressure, heart rate, and pain scores. Prevent complications such as delirium, nausea, and vomiting.
Ensure the patient meets post-anesthesia care unit (PACU) discharge criteria before returning to the ward.
8.3 Postoperative Pain Management
Administer preventive analgesics; patient-controlled analgesia (PCA) is recommended.
If pain control is inadequate, reassess the patient’s condition and adjust the analgesic plan.
9. Reversal of General Anesthetic Drugs
Reversal of Benzodiazepines: Use flumazenil (0.2–0.4 mg IV), but only when necessary.
Reversal of Opioids:
Naloxone: 0.1–0.2 mg slow IV infusion.
Nalmefene: Initial dose of 0.25 μg/kg; additional doses can be given every 2–5 minutes as needed.
Note: Opioid antagonists may cause increased pain, hypertension, and acute pulmonary edema. Use them with caution.
Part 6 | Common Adverse Reactions and Management
Intratumoral chlorine dioxide injection therapy may cause various adverse reactions. Prompt recognition and management are critical to ensure patient safety. Below are common adverse reactions and their corresponding treatment measures:
(1) Hypoxemia Management
Hypoxemia is a common complication during anesthesia and postoperative recovery, requiring urgent intervention to prevent severe outcomes.
Tracheal Intubation and Artificial Ventilation:
Immediately establish an artificial airway and initiate mechanical ventilation to correct hypoxemic states.
Correction of Hypercapnia:
Most patients with hypoxemia also have hypercapnia. Over-ventilation should be used to correct this condition. Blood gas analysis and electrolyte testing should be performed to adjust any acid-base imbalances and electrolyte disturbances.
Cerebral Resuscitation:
Hypoxemia may cause brain injury. The following actions are recommended:
Administer corticosteroids (e.g., methylprednisolone or dexamethasone injection) to stabilize cell membranes and prevent cerebral edema.
Check pupil reactions and, if necessary, administer mannitol for dehydration therapy.
(2) Local Anesthetic Toxicity
Accidental intravascular injection or excessive dosing of local anesthetics may lead to systemic toxic reactions.
Clinical Manifestations:
Central Nervous System Symptoms: Agitation, dizziness, tinnitus, perioral paresthesia, seizures, or loss of consciousness.
Cardiovascular Symptoms: Hypertension, bradycardia, ventricular tachycardia, or ventricular fibrillation.
Preventive Measures:
Avoid intravascular injection of local anesthetics; follow standardized procedures during administration.
Use ultrasound guidance for nerve blocks to ensure accurate placement of the needle relative to the nerve.
Utilize the lowest effective concentration and dose of local anesthetics; avoid excessive use of additives.
Conduct postoperative follow-ups to detect early signs of nerve injury.
Treatment Measures:
Minor nerve injuries may resolve on their own and can be supplemented with corticosteroids, vitamin B12, and physiotherapy.
Severe nerve damage (e.g., nerve compression from hematoma or nerve transection) may require surgical exploration.
(3) High-Level Block or Total Spinal Anesthesia
During epidural analgesia, accidental entry of local anesthetics into the subarachnoid space may result in a high-level block or total spinal anesthesia.
Clinical Manifestations:
Patients may exhibit agitation, severe hypotension, respiratory distress, aphonia, or loss of consciousness.
Management Principles:
Maintain circulatory and respiratory function:
Perform immediate tracheal intubation and artificial ventilation if the patient loses consciousness.
Increase fluid infusion rates.
Use vasopressors to stabilize blood pressure.
(4) Cardiac Arrest: Prevention and Management
During intratumoral injection therapy, cardiac arrest may occur due to vagal reflexes triggered by pain or tissue traction, or from hypoxia and carbon dioxide accumulation.
Types:
Reflex Cardiac Arrest: Has a generally favorable prognosis if addressed promptly.
Hypoxic Cardiac Arrest: Can lead to severe consequences, including permanent brain damage.
Preventive Measures:
Adequately control pain and avoid painful stimuli.
Perform procedures gently to prevent vagal reflexes.
Anesthesiologists should closely monitor vital signs and immediately stop procedures at the first sign of abnormalities, initiating cardiopulmonary resuscitation (CPR) as needed.
(5) Nausea and Vomiting
Nausea and vomiting may arise from the following:
Side effects of opioid medications.
Hypotension following analgesia.
Delayed gastric emptying or pain stimuli.
Management Measures:
Measure blood pressure immediately and correct any hypotension.
Administer antiemetics, such as metoclopramide or 5-HT3 receptor antagonists (e.g., ondansetron).
Prevention of Postoperative Nausea and Vomiting (PONV) for High-Risk Patients:
Combine medications with different mechanisms to prevent PONV.
Recommended Drugs: Corticosteroids (e.g., dexamethasone), 5-HT3 receptor antagonists (e.g., ondansetron, granisetron), NK-1 receptor antagonists (e.g., aprepitant), or benzamide derivatives (e.g., metoclopramide).
Part 7 | Summary and Recommendations
Pain management and complication handling in intratumoral chlorine dioxide injection therapy should prioritize patient safety. Through strict pre-treatment evaluations, standardized anesthesia practices, and diligent postoperative monitoring, the incidence of adverse reactions can be significantly reduced. This ensures improved treatment outcomes and higher patient satisfaction.





What is the PPM range of these chlorine dioxide injections?