9. Guidelines for Intratumoral Injection of Chlorine Dioxide in Breast Cancer
9.1 Indications
9.1.1 Breast cancer at any stage.
9.1.2 Recurrence after radical mastectomy or chemoradiotherapy.
9.1.3 Metastasis to the breast pectoralis or supraclavicular lymph nodes.
9.1.4 Distant organ metastasis from breast cancer.
9.1.5 Normal bleeding and clotting times.
9.2 Contraindications
9.2.1 Platelet count < 60×10⁹/L.
9.3 Pre-treatment Preparation
9.3.1 Complete pre-treatment examinations:
Blood count and coagulation tests.
Liver and kidney function tests.
Tumor markers: ER, PR, HER2, CA153, CEA.
Imaging: Mammography, breast ultrasound, abdominal and neck/axillary ultrasound, CT of the chest and brain.
Histopathological diagnosis.
9.3.2 Discuss the procedure and obtain consent, addressing possible complications such as fever, local skin ulcers, and pain.
9.3.3 Administer intramuscular injections of 0.1g butorphanol, 1KU antihemorrhagic agent, and 4-8mg ondansetron 15 minutes before treatment.
9.4 Puncture Needle and Auxiliary Equipment
Use a 23G×15.0 cm or 25G×9.0 cm puncture needle, syringe, 10ml and 20ml syringes, and ultrasound or CT machine.
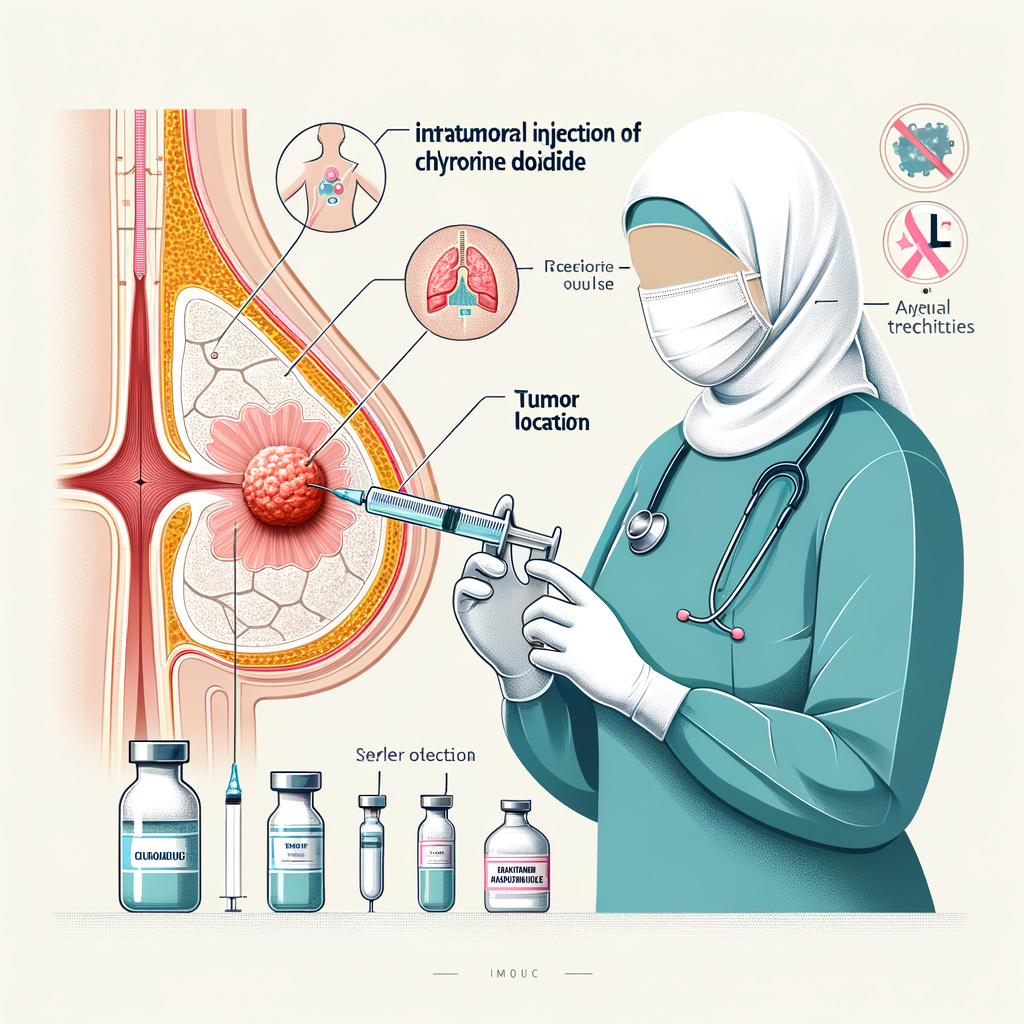
9.5 Treatment Procedure
9.5.1 Select the puncture site under ultrasound or CT guidance.
9.5.2 Disinfect the skin, wear sterile gloves, and cover with a sterile drape.
9.5.3 Use 2% lidocaine for local anesthesia, stabilize the puncture site with the left hand, and insert the needle into the tumor center with the right hand.
9.5.4 Withdraw the needle core, connect a high-pressure syringe, and slowly inject chlorine dioxide solution under ultrasound or CT guidance. For tumors ≤4 cm, use single-point injection based on 30% of the tumor volume (ml). For tumors >4 cm, use multi-point injections with the same volume calculation.
9.5.5 After injection, remove the needle, cover with sterile gauze, and secure with tape.
9.5.6 Observe for 5–10 minutes post-procedure and then send the patient back to the ward.
9.6 Post-treatment Care
9.6.1 Monitor for redness, swelling, ulceration, pain, fever, and fatigue, and treat any complications promptly.
9.6.2 Check blood counts twice weekly, monitor WBC and PLT changes. Use granulocyte colony-stimulating factor if WBC < 4.0×10⁹/L, interleukin-11 for PLT < 80×10⁹/L, and oral iron supplements for Hb < 80g/L; transfusion if Hb < 60g/L.
9.6.3 Conduct weekly liver and kidney function tests to check for any damage.
9.6.4 After one treatment cycle, assess peripheral blood T lymphocyte subsets (CD4, CD8), and relevant molecular biological markers and genes.
9.7 Experience and Complications Management
9.7.1 For local redness, heat, and pain, provide anti-infective treatment and magnesium sulfate wet compress to reduce swelling.
9.7.2 For exudation or ulceration, apply ozone treatment locally to keep the wound clean and dry.
9.7.3 Skin ulceration post-treatment can occur due to drug leakage or shallow puncture. Choose puncture points near the base to avoid irritating the skin. Post-treatment, apply a local wet compress to reduce congestion and edema.


Dr. Liu,
Thank you for sharing your research and insights. As a stage 4 breast cancer survivor who was given only months to live by UCLA—and whose cancer had spread to my bones and all lobes of my lungs—I was blessed to find complete healing through Dr. Payán at CMN Hospital. I’ve been cancer-free since 2011, without ever having chemotherapy.
Dr. Liu,
I received an email from one of the women I coach on the emotional part of the cancer journey. She sent me the link to your Guidelines for Intratumoral Injection of Chlorine Dioxide in Breast Cancer, which led me to your X account and the article you posted here:
https://x.com/liuxuewu/status/1929449724991750266?t=_8xJaiERk5BtpuKgc5CjMw&s=19
I had previously heard of other intratumoral therapies like lactic acid and ozone, but now intratumoral chlorine dioxide has come up twice in just the last week. It’s not uncommon for women I coach to bring forward new therapies and ask for my non-professional thoughts. It’s always a slippery slope, since I am not a doctor or in a position to advise—only to listen, ask questions, and share information with care and caution.
Since these women are in advanced stages, I think it's safe to assume that the answer you share with me could help many others as well.
Would you be willing to share whether or not your protocol can help women with stage 4 cancer that has metastasized throughout the body—to the bones, brain, lungs, and other vital organs—along with a link that explains this clearly? That way, I’m not positioned as the voice of understanding or advice, and I can direct them to accurate information directly from you.
Thank you so much for your time and consideration. I truly appreciate the work you're doing and the hope it brings to so many.
Sincerely,
Shannon Knight
Great info! We have a breast cancer patient here (Philippines) who is looking at doing this already. What concentration should this be? 3000ppm? Our Chlorine dioxide community (with some doctors) will help/guide her through. There is no mention of the concentration of Chlorine dioxide to be used.