Comparing Medical Freedom: Germany vs. Japan vs. the United States
The Need to Expand Medical Freedom for Americans
Recently, while promoting my intratumoral chlorine dioxide injection therapy, I noticed that the clinics willing to collaborate with me are mostly located in countries with high levels of medical freedom. These include less developed countries, such as Mexico, Brazil, and the Philippines, where the high degree of medical freedom may stem from the limited capacity of their healthcare systems, allowing residents to make medical choices that exceed the system's capabilities. There are also developed countries, such as Germany and Japan, where medical freedom is similarly high.
Similarities Between Germany’s Healthcare System and Japan’s
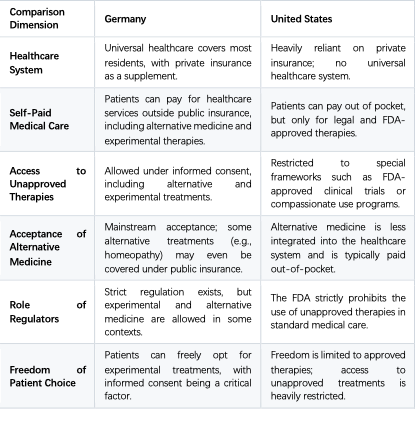
Germany’s healthcare system is in many ways similar to Japan’s, particularly in allowing patients the freedom to choose unapproved or experimental therapies. While Germany also has strict regulations, the overall system provides greater flexibility for patients to voluntarily pursue certain unapproved or alternative treatments (provided informed consent is given). Below is a detailed analysis of the similarities between Germany and Japan, as well as the differences with the U.S.
I. Key Characteristics of Germany’s Healthcare System
1. Germany’s Universal Healthcare System
Germany has a universal healthcare system (Gesetzliche Krankenversicherung, GKV) that covers the vast majority of its residents.
Similar to Japan, Germany’s public insurance system includes coverage for most standard medical services, while additional out-of-pocket services can be accessed through optional private healthcare choices.
Germany allows private insurance (Private Krankenversicherung, PKV) as an option for high-income individuals or those seeking premium services.
2. Legal Self-Paid Healthcare and Alternative Medicine
In Germany, patients can opt for additional out-of-pocket healthcare services outside the standard coverage of public insurance, known as Individuelle Gesundheitsleistungen (IGeL), which may include non-standard or alternative medicine.
Medical institutions may provide experimental or unapproved therapies (such as homeopathy or alternative treatments) as long as the patient gives informed consent.
This flexibility aligns closely with Japan’s concept of "自由診療" (free medical care or self-paid medical care).
3. Emphasis on Patient's Autonomy
Germany’s healthcare standards place a strong emphasis on patient autonomy(Patientenautonomie), where laws explicitly protect the right of patients to make choices about their own medical care.
Physicians are required to provide patients with comprehensive and transparent information so that they can make an informed decision. As long as a therapy follows legal and ethical codes, patients are allowed to pursue that treatment.
4. High Acceptance of Alternative and Experimental Therapies
Germany is known for its openness to alternative medicine (such as homeopathy, traditional Chinese medicine, and herbal treatments), with many clinics and private medical institutions offering such services.
For experimental therapies or unapproved treatments, they can often be provided as long as the patient is fully informed of the risks and gives explicit consent.
II. Germany vs. Japan: Similarities in Their Healthcare Systems
1. Both Have Universal Healthcare Systems
Both Germany and Japan operate universal healthcare systems that ensure accessibility to healthcare services at relatively low costs.
In both countries, patients have the option to pay out of pocket for additional medical services that are not covered by public insurance.
2. Self-Paid Medical Services Are Available in Both Systems
In Japan, patients can opt for "自由診療" (free medical care), paying for treatments not covered by public insurance.
In Germany, patients can access non-insured services through the IGeL system or private insurance, which often includes alternative or experimental treatments.
3. Emphasis on Patient’s Medical Choice
Japan's Constitution Article 13 guarantees “the rights to life, liberty, and the pursuit of happiness,” and this has been interpreted to include the right to medical freedom.
Similarly, Germany’s Patients’ Rights Law (Patientenrechtegesetz) and its Basic Law (Grundgesetz) safeguard patients’ autonomy, enabling them to make informed decisions regarding their treatment.
4. Both Allow Space for Unapproved Therapies
Japan allows unapproved therapies through its framework of "free medical care" or special clinical research, provided regulatory and ethical requirements are followed.
Germany permits experimental therapies or treatments not yet universally approved if patients give informed consent in accordance with laws and ethics.
III. Germany vs. the United States: Differences in Healthcare Systems
IV. Germany’s Position on Unapproved Therapies
1. Legitimacy of Alternative Medicine
Germany has a high level of acceptance for alternative medicine (such as homeopathy, herbal medicine, and traditional Chinese treatments). Many clinics and private institutions actively offer such therapies.
In some cases, alternative treatments may even be partially reimbursed under public insurance.
2. Providing Experimental Therapies
Physicians in Germany may offer unapproved or experimental treatments if patients are fully aware of the risks, are given transparent information, and provide informed consent.
For example, cancer patients in Germany may access experimental therapies or participate in specialized care programs offered by hospitals.
3. Legal and Ethical Boundaries
Germany’s Patients’ Rights Law requires that informed consent must be secured for any medical treatment, particularly experimental or unapproved therapies.
Physicians must ensure that treatments do not pose excessive risks to the patient and must comply with medical and ethical standards.
V. Medical Freedom: Germany vs. Japan vs. the U.S.
Conclusion: Is Germany Like Japan?
Yes, Germany’s healthcare system shares many similarities with Japan’s, especially in terms of medical freedom.
1. Similarities Between Germany and Japan
Both countries boast universal healthcare systems offering comprehensive coverage, while also enabling patients to access supplemental or experimental services outside the standard framework.
Informed consent is a critical part of both systems, allowing unapproved therapies to be provided legally under specific conditions.
2. Differences From the U.S.
Unlike in the U.S., where the FDA’s strict regulations drastically limit access to unapproved therapies, Germany and Japan adopt relatively flexible approaches toward experimental and alternative treatments.
Patients in Germany and Japan enjoy broader freedom to choose alternative medicine or experimental therapies, especially in out-of-pocket arrangements, compared to the heavily regulated U.S. system.
From the perspective of medical choice freedom, Germany aligns more closely with Japan, while the United States appears more restrictive due to FDA oversight.





Well done, Xuewu to challenge the medical unfreedom in the U.S.
If you want to know the why and how the Medical Mafia and the pHarmaceutical Cult came together to erect the current Medical Penitentiary and Iatrogenic Kill Machine go here among others:
EPISODE 286 – ROCKEFELLER MEDICINE by Corbett | Nov 2, 2013
https://corbettreport.com/episode-286-rockefeller-medicine/
THE FLEXNER REPORT: HOW JOHN D. ROCKEFELLER USED THE AMA TO TAKE OVER WESTERN MEDICINE
https://thefreedomarticles.com/amp/flexner-report-rockefeller-ama-takeover/
JOHN ROCKEFELLER: HOW HE TOOK CONTROL OVER MODERN MEDICINE
https://hannenabintuherland.com/usa/john-rockefeller-how-he-took-control-over-modern-medicine/
DEATH BY MEDICINE by Gary Null 2010
https://archive.org/details/DeathByMedicine/page/n15/mode/1up
ON IATROGENIC OR MEDICALLY CAUSED PREMATURE DEATH—
2nd Or 3rd Leading Cause Of Death In Welfare State Countries Worldwide
http://www.huffingtonpost.com/allen-frances/why-are-medical-mistakes-_b_5888408.html
Some push back:
THE ASSOCIATION OF AMERICAN PHYSICIANS AND SURGEONS (AAPS) – is a non-partisan professional association of physicians in all types of practices and specialties across the country. Since 1943, AAPS has been dedicated to the highest ethical standards of the Oath of Hippocrates and to preserving the sanctity of the patient-physician relationship and the practice of private medicine.
https://aapsonline.org/about-aaps/
NATIONAL HEALTH FREEDOM ACTION
The Mission
To ensure that the people of this nation have access to the broad domain of healing and health care information and services, to ensure the right of practitioners of the healing arts to practice, and to educate the public, promote health and well-being, conduct surveys and research, and participate in legislative, regulatory, legal, or public policy-reform and lobbying to accomplish the goal of health freedom.
https://nationalhealthfreedomaction.org/advocacy-info/
Get free, stay free.
The hope for a transformation in medical freedom in the United States rests with Robert F. Kennedy Jr.
The method for this change is very simple: have the FDA advocate for medical practices to adhere to Article 37 of the Helsinki Declaration.
Without changing current regulations, it may be challenging for the FDA to directly advocate for Article 37 of the Helsinki Declaration, but it is possible to align with its principles depending on how it is interpreted and implemented.
What is Article 37 of the Helsinki Declaration?
Article 37 primarily states that when no effective treatment exists, doctors, after thorough consideration, may use unproven or experimental treatments to help patients, provided that the patient's informed consent is obtained. This provision aims to protect patients' rights while allowing innovative treatments in emergency or special circumstances.
Background on Current FDA Regulations
In the United States, the FDA's main responsibility is to ensure the safety and efficacy of drugs and medical devices. Under current regulations, unapproved drugs typically need to go through clinical trial procedures and final approval before they can be legally used for patient treatment. While there are stringent restrictions on the use of unproven drugs, the FDA has established certain exceptions, such as:
Expanded Access Programs: Allowing the use of unapproved drugs under specific conditions for patients who meet the criteria.
Compassionate Use: Providing experimental treatments for eligible patients in dire situations.
Possibilities for the FDA to Advocate Article 37
To promote the principles of Article 37 of the Helsinki Declaration, the FDA could take the following actions within the current regulatory framework:
Enhance Expanded Access and Compassionate Use Programs
The FDA could actively promote these existing channels, enabling more patients to access unproven but potentially life-saving treatments.
Encourage Physicians to Follow the Principles
The FDA could incorporate the ideas articulated in Article 37 (e.g., trying experimental therapies in the absence of treatment options) into guidelines for physicians, particularly in extreme or emergency situations.
Strengthen Informed Consent Processes
Even when experimental treatments are used, the FDA could reinforce the importance of obtaining informed consent to ensure compliance with the ethical principles outlined in Article 37.
Support Related Research and Public Dialogue
The FDA could encourage the medical community, academic researchers, and industry groups to discuss Article 37 of the Helsinki Declaration, raise awareness, and optimize existing regulations accordingly.
Challenges and Limitations
Legal Conflicts
Fully adopting Article 37’s principles by relaxing regulatory oversight on experimental drugs may conflict with current laws, especially if robust clinical data on the safety and efficacy of those treatments is not yet available.
Safety and Ethical Concerns
Advocating for Article 37 could raise safety concerns, particularly when using treatments that lack sufficient validation.
Conflict of Interests and Liability
Allowing broader use of experimental treatments could lead to abuse by pharmaceutical companies or expose patients to high medical risks.
Conclusion
Without changing current regulations, the FDA could reflect and advocate for the spirit of Article 37 through measures such as promoting expanded access or compassionate use policies, reinforcing informed consent principles, and supporting discussions on medical ethics. However, systematic and broader implementation of Article 37’s principles may still require adjustments to existing regulations.