New Discovery in Cancer Treatment: The Relationship Between CEA Levels and Intratumoral Injection of Chlorine Dioxide Therapy
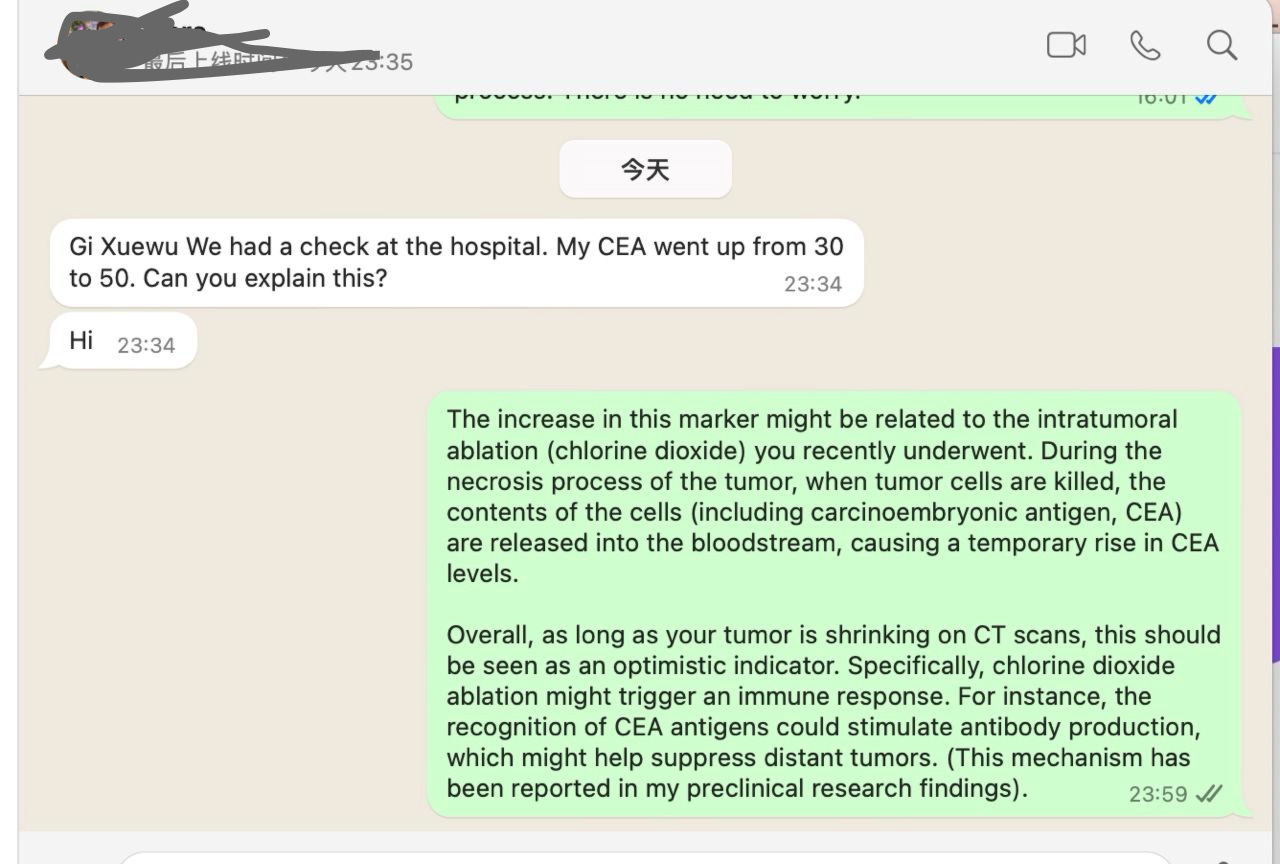
Yesterday, a cancer patient in Germany who had received two intratumoral injections of chlorine dioxide contacted me. His tumor showed significant shrinkage in imaging studies, but he raised an important question: "Gi Xuewu, we had a check at the hospital, and my CEA went up from 30 to 50. Can you explain this?"
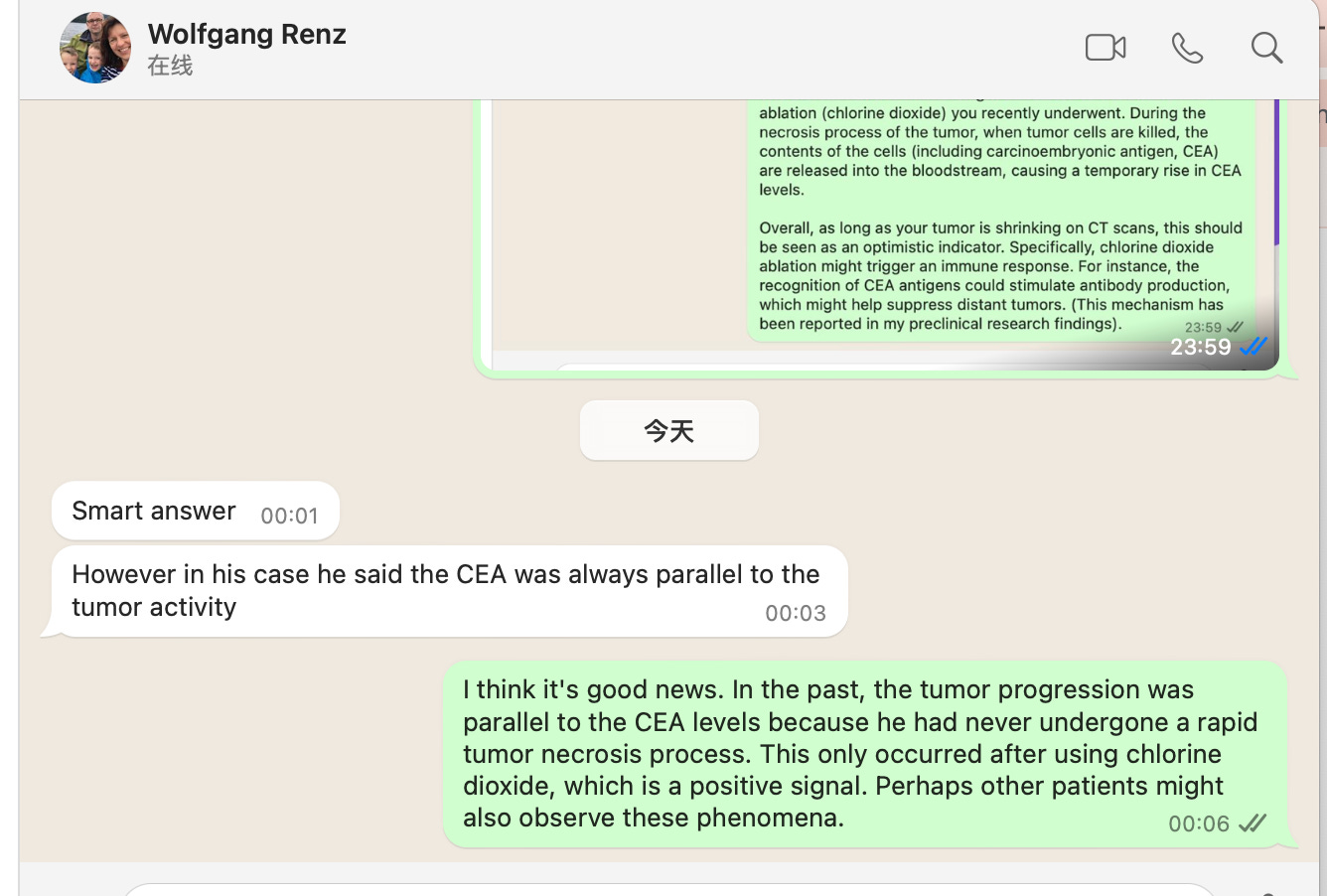
I explained to him that this phenomenon might be due to rapid tumor necrosis, which resulted in the release of large amounts of carcinoembryonic antigen (CEA) into his bloodstream. This explanation was also supported by Dr. Wolfgang in Germany, who mentioned: "The patient's CEA has always been parallel to tumor activity."
After a more detailed analysis, the most reasonable explanation for this phenomenon is as follows: In the past, the patient's tumor remained stable or progressed slowly, and the CEA levels corresponded to the tumor dynamics accordingly. However, this time, the chlorine dioxide injection caused massive tumor necrosis. The large amount of CEA released into the bloodstream is a positive signal, indicating that the tumor breakdown is truly happening. In other words, the increase in CEA does not indicate worsening but rather signifies that the treatment is working.
CEA Elevation Due to Rapid Tumor Necrosis: A Potential Marker for Treatment Success
In my research and observations, I found that similar phenomena have been reported with other treatments. For instance, after ablation therapies (such as radiofrequency ablation or cryoablation), patients' CEA levels often show a significant temporary increase. This elevation is attributed to the release of tumor antigens, including CEA, into the bloodstream due to cell necrosis.
This realization leads me to believe that CEA could be a valuable biomarker for evaluating the efficacy of intratumoral chlorine dioxide therapy. Although imaging studies like CT or ultrasound are effective in observing tumor necrosis immediately after treatment, some tumors may show delayed changes in imaging. However, an immediate rise in CEA levels may serve as a more direct and early indicator of treatment effectiveness.
CEA: The Significance of a Tumor Biomarker
What Is CEA?
CEA (Carcinoembryonic Antigen) is a common tumor marker primarily found in fetal tissue during development. In healthy adults, it is either absent or present in minimal amounts. Elevated CEA levels are often associated with the presence and progression of specific cancers, making it a widely used marker for cancer monitoring.
Major Applications of CEA:
Auxiliary Diagnosis:
CEA alone cannot diagnose cancer, but elevated levels may suggest the need for further testing for conditions such as colorectal cancer, lung cancer, gastric cancer, breast cancer, and others.Monitoring Treatment Effectiveness:
After cancer treatments, a decrease in CEA levels generally indicates effective therapy. Conversely, a rise might suggest recurrence or progression.Assessing Prognosis:
Dynamic changes in CEA levels can reflect tumor burden and serve as an important parameter for follow-up evaluations.
Causes of Elevated CEA:
Cancer-related:
Commonly elevated in colorectal cancer, lung cancer, gastric cancer, breast cancer, and others.Non-cancer-related:
Smoking, inflammation, and liver conditions can also cause elevated CEA levels. Hence, clinical context must be considered when interpreting changes.
Why CEA Levels May Rise After Tumor Ablation
During treatment, especially after tumor ablation or intratumoral injection procedures, a temporary rise in CEA is a frequently observed phenomenon. The reasons include:
Release of Antigens Due to Tumor Cell Necrosis:
Tumor cells undergo necrosis during treatment, releasing their internal contents, including CEA, into the bloodstream. This causes a short-term and significant rise in CEA levels.Inflammatory Response:
Ablation or injection treatments can cause localized tissue damage and inflammation, which may further trigger the release of CEA.Immune System Involvement:
The immune system plays a role in clearing necrotic tumor cells and their associated antigens, leading to a peak in CEA before the antigen levels normalize as they are metabolized by the liver.
Based on my experience, I attempted to explain why intratumoral chlorine dioxide injection led to such a sharp increase in CEA levels and hypothesize the significance of this elevation.
The Unique Mechanism of Chlorine Dioxide Injection: Rapid Necrosis and Immune Activation
1. Rapid Necrosis: The Direct Effect of Treatment
Chlorine dioxide, as a strong oxidizing agent, can rapidly destroy tumor cells, leading to necrotic cell death. This "rapid necrosis" not only results in tumor shrinkage but also releases a substantial amount of tumor antigens (such as CEA) into the bloodstream.
2. Immune Activation: The Indirect Effect of Treatment
Following tumor cell death, the released antigens (such as CEA) may be recognized by the immune system, which then activates immune cells such as dendritic cells and T cells. This immune response may not be limited to the primary treatment site but may also inhibit distant tumor lesions.
3. "Vaccine Effect": A Systemic Anti-Tumor Response
In tumor immunology, this process is referred to as the "vaccine effect," wherein the release of tumor antigens due to necrosis triggers an immunological response akin to vaccination. This response may lead to systemic anti-tumor activity, offering protective effects even against metastatic lesions.
How to Observe and Evaluate Treatment Effectiveness
1. Monitoring CEA Dynamics:
A short-term rise in CEA levels is a normal biological response post-treatment. The key is to observe its subsequent trend.
If CEA levels gradually decrease after the initial peak, it is typically a favorable indicator of treatment efficacy.
2. Imaging Studies:
Follow-up imaging (e.g., CT or MRI) is the gold standard for evaluating tumor volume reduction and necrosis extent. Persistent shrinkage or indistinct tumor boundaries signal effective therapy.
3. Immune Response Assessment:
Monitoring the activity of immune cells (e.g., activated T cells, NK cells) or tumor-specific antibody levels in the blood may provide insights into the enhanced anti-tumor immune response triggered by the treatment.
Future Prospects: CEA as a Marker for Treatment Efficacy
This experience made me realize that the dynamic changes in CEA levels might be an important biomarker to evaluate the success of intratumoral chlorine dioxide therapy. If more cases demonstrate a strong correlation between CEA levels and therapy outcomes, I plan to include CEA as a standardized parameter in clinical treatment evaluation.
Additionally, I aim to explore other potential biomarkers to further refine the monitoring system for treatment efficacy. This not only enhances treatment optimization but also provides patients with more accurate prognostic assessments.
Conclusion
A temporary CEA elevation is not necessarily bad news. After intratumoral chlorine dioxide injection, the elevation of CEA levels may reflect rapid tumor necrosis and treatment efficacy. By dynamically monitoring CEA levels and combining them with imaging studies and immunological assessments, we can make a more comprehensive evaluation of the treatment's success. This experience offers valuable insights for future clinical practice.