Why is Tumor Ablation the Best Approach for Cancer Treatment
Why is Intratumoral Injection of CD the Most Effective Method?
Using a first principles-based cancer treatment analysis model, we have identified five key factors that significantly influence the ultimate goal of cancer treatment: extending the patient's high-quality survival period. This new assessment method allows us to reevaluate existing cancer treatments to ensure they not only prolong survival but also enhance the quality of life for patients. The evaluation considers long-term effects rather than just short-term outcomes.
With this new assessment framework, we can offer truly meaningful treatment options tailored to individual patients. A major advantage is that this method aligns with the existing medical system, as the recommended treatment plans still adhere to NCCN guidelines. Consequently, cancer patients can actively participate in the decision-making process of their treatment plans, enabling doctors to provide more personalized care.
To further guide cancer patients in applying these principles during their cancer journey, we will conduct a detailed analysis and evaluation of six common treatment plans. This comprehensive analysis provides cancer patients with detailed insights into each treatment method, including their benefits, limitations, and applicable conditions. Such information aids both patients and doctors in making joint decisions, selecting the treatment plan that best matches the patient's condition and quality of life needs.
The application of this assessment method not only makes the treatment process more transparent but also enhances patient autonomy, allowing participation in treatment decisions based on individual situations and preferences. This personalized treatment strategy is ultimately more beneficial for patients' long-term health and quality of life.
In this context, tumor ablation emerges as the best approach to optimizing the high-quality survival period for cancer patients. It effectively eliminates cancer cells while minimizing harm to the body, thus supporting a longer, healthier life.
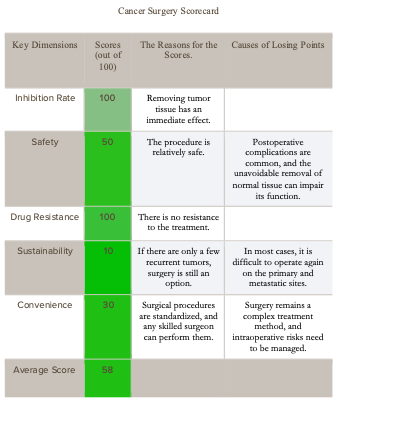
1. Cancer Surgery
Surgical treatment is a common method for addressing cancer, primarily aimed at removing tumors and potential cancer cells to reduce or eliminate the disease within the patient's body. The type, scope, and complexity of the surgery vary depending on the type, location, stage of the cancer, and the patient's overall health. Common surgical approaches include excision, which involves removing the tumor and some surrounding normal tissue, and radical surgery, which aims to completely remove the source of the cancer and associated lymph nodes.
Pre-surgical preparation includes a comprehensive physical examination, blood tests, and imaging studies to assess the patient's health status and surgical risks. Doctors discuss the potential risks and expected outcomes with patients to ensure they fully understand the necessity and possible consequences of the surgery. Surgery is usually performed under general anesthesia, and surgeons, depending on the type and purpose of the surgery, precisely remove the tumor and potentially affected surrounding tissues. High-precision tools like microscopes and endoscopes may be used to enhance the accuracy and safety of the procedure.
During the post-surgery recovery period, patients may need to stay in the hospital for observation. Doctors closely monitor the patient's recovery and potential complications, which may include pain management, wound care, and physical therapy. Like all surgeries, cancer surgery carries certain risks and potential complications, such as infection, bleeding, anesthesia-related risks, and long-term physiological or psychological effects. Doctors assess these risks based on the patient's specific conditions and take appropriate preventive measures.
If the cancer is in an early stage, surgical treatment often dominates due to its ability to remove all visible tumor tissue at once, achieving the highest rate of suppression. In the short term, because of its direct and rapid effects, surgical treatment is often the easiest option for patients to accept.
Following the principle of "early detection, early treatment," doctors and patients usually prefer surgical treatment; however, if the cancer has progressed to an intermediate stage, the conventional approach is to first use chemotherapy or targeted drugs to shrink the tumor, followed by surgical removal. The table below evaluates general cancer surgical treatments based on five factors.
2. Chemotherapy
Chemotherapy is a common method for treating cancer, utilizing chemical drugs to attack and destroy cancer cells. These drugs can block the growth and division of cancer cells, helping to shrink tumors, control the spread of the disease, or alleviate symptoms. Chemotherapy can be used alone or in combination with surgery, radiation, or other treatments to enhance its effectiveness.
There is a wide variety of chemotherapy drugs, each with its specific mechanism of action. Some drugs may damage the DNA of cancer cells, preventing them from replicating and growing; others may interfere with specific proteins or signaling pathways that cancer cells use to grow. Doctors select the most appropriate drug or combination of drugs based on the patient's condition and type of cancer.
Chemotherapy can be administered in various ways, including orally, by intravenous injection, or directly into body cavities like the cerebrospinal fluid. The frequency and duration of treatment depend on the specific drugs and the treatment plan, which could be daily, weekly, or monthly.
Despite its key role in controlling cancer, chemotherapy can cause a range of side effects. These are primarily due to the fact that chemotherapy drugs affect not only cancer cells but also normal cells that divide rapidly, such as blood cells, cells in the digestive tract, and hair follicle cells. Common side effects include hair loss, nausea, vomiting, fatigue, loss of appetite, and mouth sores. The severity of these side effects varies from person to person, and many can be managed with medication and other treatment approaches.
During chemotherapy, patients need regular blood tests and other physical examinations to monitor their body's response to the treatment. This monitoring helps in timely adjusting the treatment plan to address potential complications or side effects, ensuring the safety and effectiveness of the treatment. Overall, chemotherapy is a complex process that requires close cooperation and coordination among doctors, patients, and their families to achieve the best treatment outcomes and quality of life.
Compared to surgical treatment, the inhibitory effects of chemotherapy are generally lower, and it is associated with more side effects. This is mainly because chemotherapy drugs are not delivered directly to the tumor tissue but are administered systemically. Since the tumor tissue constitutes only a small part of the body, the effective concentration of chemotherapy drugs at the tumor site is significantly reduced. Increasing the drug concentration to improve effectiveness can lead to more systemic side effects. The following table evaluates five common factors of chemotherapy.
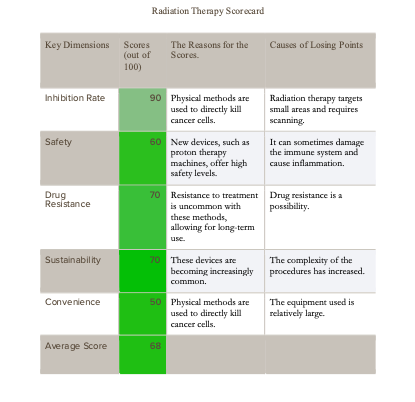
3. Radiation Therapy
Radiation therapy is a common medical technique used to treat cancer, employing high-energy radiation to kill cancer cells or inhibit their growth. This treatment can be delivered using external beam radiation machines or by placing radioactive substances directly near the tumor inside the body. It can be the primary treatment method or used in conjunction with chemotherapy, surgery, and other treatments to enhance effectiveness.
Before starting radiation therapy, doctors utilize advanced imaging technologies like CT scans, MRI, or PET scans to pinpoint the exact location and size of the tumor. This information helps radiation therapists accurately target the treatment area, minimizing damage to surrounding healthy tissue. The treatment plan is usually developed by a team of specialists, including radiation oncologists, medical physicists, and radiation therapists.
During radiation therapy, patients lie on a specially designed table and must remain still. The treatment machine moves around the patient, emitting radiation from multiple angles to precisely target the tumor. Each session typically lasts only a few minutes, though the entire treatment course may span several weeks, depending on the type and purpose of the treatment.
The goal of radiation therapy is to destroy as many cancer cells as possible while protecting the surrounding normal cells. To achieve this, treatments are often divided into multiple small doses, administered daily, allowing normal cells the chance to repair damage incurred during treatment. This approach of fractionating the dose helps improve the safety and effectiveness of the treatment.
Despite its high precision, radiation therapy can still affect surrounding normal tissues. During and after treatment, patients may experience side effects such as skin irritation, fatigue, and hair loss. The severity of these side effects varies from person to person and usually subsides after treatment ends.
With technological advancements, radiation therapy has become more precise and effective. Techniques like Intensity-Modulated Radiation Therapy (IMRT) allow doctors to adjust the intensity of the radiation beams, targeting the tumor more precisely while protecting more normal tissue. Another technique, Stereotactic Body Radiation Therapy (SBRT), enables precise targeting of small or well-defined tumors with higher doses in fewer sessions.
Over the past 50 years, significant progress has been made in radiation therapy technology, achieving a favorable balance between inhibition rates and side effects. However, the complexity and high cost of radiation therapy equipment remain drawbacks, making its use less convenient. The following table provides a detailed assessment of five key factors of radiation therapy.
4. Targeted Therapy
Targeted drug therapy is a modern cancer treatment method that uses drugs to precisely attack specific molecular mechanisms in cancer cells, rather than affecting both healthy and cancerous cells as traditional chemotherapy does. This approach is based on a deep understanding of cancer biology, particularly the molecular and cellular processes involved in cancer cell growth and spread.
The development of targeted therapy drugs begins with the identification of biological markers unique to cancer cells. These biomarkers are specific proteins or genes that cancer cells rely on for survival and proliferation. By targeting these key biomarkers, targeted drugs can block the growth signals of cancer cells or alter their growth environment, thereby inhibiting tumor development.
A key advantage of targeted therapy is its selectivity, which allows the drugs to act more directly on cancer cells, reducing damage to normal cells. This typically results in fewer side effects compared to traditional chemotherapy, thus improving patients' quality of life.
A common form of targeted therapy involves the use of small molecule inhibitors. These drugs can enter cells and directly target specific proteins or signaling pathways. For example, some small molecule inhibitors block tyrosine kinases, enzymes that play a crucial role in the proliferation of many cancer cells.
Another type of targeted therapy involves monoclonal antibodies. These large molecule drugs are designed to recognize and bind to specific molecules on the surface of cancer cells, thereby triggering the immune system to attack the cancer cells or directly blocking the growth signals of the cancer cells. Monoclonal antibodies can also be engineered to carry toxins, radioactive substances, or other drugs, delivering these destructive agents directly to cancer cells without affecting surrounding healthy cells.
The effectiveness of targeted drug therapy depends on various factors, including the type of cancer, individual differences among patients, and the genetic characteristics of the tumor. Therefore, doctors may recommend molecular diagnostic testing before starting treatment to identify the specific genetic and molecular features of the tumor, enabling the selection of the most appropriate targeted drug.
Advances in cancer genomics and molecular biology are continually leading to the discovery and development of new targets and therapeutic drugs. These advancements not only increase the available treatment options but also enhance the precision and personalization of treatments.
In practice, targeted drug therapy can be used alone or in combination with other cancer treatments such as chemotherapy, radiation therapy, or immunotherapy. This multimodal treatment strategy aims to attack cancer from different angles to enhance therapeutic effects while minimizing the impact on the patient.
Although targeted drug therapy is highly effective in many cases, it also faces challenges such as the development of drug resistance. Cancer cells can mutate over time, developing resistance to targeted drugs, which necessitates ongoing adjustments and optimizations of treatment plans.
Targeted drugs generally show advantages over chemotherapy drugs in terms of short-term inhibition rates and safety, but due to significant drug resistance, their sustainability scores are lower. The following table provides a detailed assessment of five key factors of targeted therapy.
5. Tumor Ablation
Tumor ablation is a minimally invasive treatment for certain types of tumors, particularly in the liver, kidneys, and lungs. This technique destroys tumor cells by applying extreme temperatures directly, using various energy sources such as radiofrequency, microwaves, lasers, and cryotherapy. Each method has its unique approach and mechanism, but all aim to precisely eliminate tumor cells while preserving surrounding healthy tissue.
Radiofrequency ablation (RFA) uses radio waves to generate heat, destroying tumor cells by heating them to lethal temperatures. A thin needle-like electrode is inserted into the tumor, where it generates heat through electrical currents.
Microwave ablation (MWA) is similar to RFA but uses microwave energy to achieve higher temperatures and destroy tumor cells more quickly. It can treat larger tumors than RFA.
Laser ablation directs high-energy lasers at the tumor, converting light energy into heat to destroy the cells. A laser fiber is inserted through the skin into the tumor, allowing precise control over the laser's release to minimize damage to surrounding tissues.
Cryotherapy, also known as cryoablation, treats tumors by freezing the cells with extremely low temperatures. Liquid nitrogen or argon gas is delivered to the tumor through a thin probe, rapidly lowering the temperature and forming ice crystals that disrupt cell structures, leading to cell death.
Tumor ablation is usually performed under local anesthesia or mild sedation, depending on the ablation method and the size of the treatment area. During the procedure, imaging techniques like ultrasound, CT scans, or MRI may be used to guide and monitor the probe's position, ensuring energy is accurately delivered to the tumor.
Due to its minimally invasive nature, tumor ablation often results in shorter recovery times and lower risks of postoperative pain and complications compared to traditional open surgery. This makes it a viable option for patients who may not be suitable for conventional surgery.
Tumor ablation is suitable for patients with smaller, well-defined tumors and is particularly beneficial for those who cannot undergo traditional surgery due to age or other health issues. It can also serve as a supplementary treatment for patients whose tumors have not been completely removed or are at risk of recurrence.
Photodynamic therapy (PDT) is another form of tumor ablation that combines a photosensitizing agent with a specific wavelength of light. This treatment involves administering a photosensitizer to the patient, which accumulates in the tumor cells. After a period, the treated area is exposed to light of a specific wavelength. The light's energy, absorbed by the photosensitizer, generates reactive oxygen species or free radicals that destroy the tumor cells.
PDT's advantage lies in its high selectivity, primarily targeting tumor cells that have accumulated the photosensitizer, causing minimal damage to surrounding healthy tissue. Side effects are generally mild, mainly photosensitivity reactions where the patient's skin and eyes may become more sensitive to light for some time after treatment.
PDT is primarily used for treating superficial tumors, such as skin cancer and certain types of head and neck cancers. It can also be used as an adjunct treatment for some internal tumors, for example, by guiding light to specific internal sites through an endoscope. In some cases, PDT is also used to treat non-cancerous conditions like macular degeneration.
The effectiveness of PDT depends on several factors, including the type of photosensitizer, the wavelength of light, the duration of exposure, and the type and location of the tumor. As new photosensitizers and more precise light sources are developed, the scope and expected outcomes of PDT are likely to improve further.
Tumor ablation is technically similar to radiation therapy but uses smaller, more portable, and sustainable equipment, typically with fewer side effects than radiation therapy. The following table provides a detailed assessment of five key factors of tumor ablation.
6. Immunotherapy
Cancer immunotherapy is a treatment that harnesses the power of the human immune system to recognize and attack cancer cells. This therapy builds on the immune system's natural function to protect the body from diseases. Normally, the immune system can identify and eliminate abnormal cells, including cancer cells. However, cancer cells often find ways to evade immune surveillance. The goal of cancer immunotherapy is to enhance the immune system's natural functions to help it more effectively identify and attack cancer cells.
One common form of cancer immunotherapy is immune checkpoint inhibitors. Immune checkpoints are mechanisms on the immune system that prevent immune cells from attacking normal cells. Cancer cells can exploit these checkpoints to protect themselves from immune attacks. Immune checkpoint inhibitors work by blocking these checkpoints, removing the cancer cells' "invisibility cloak" and allowing the immune system to detect and destroy them. For example, PD-1 and CTLA-4 are two immune checkpoints commonly exploited by cancer cells, and inhibitors targeting these can effectively activate the immune system against cancer.
Another strategy is cell therapy, such as CAR-T cell therapy. This involves extracting T cells (a type of immune cell) from the patient's body, genetically modifying them in the laboratory to better recognize cancer cells, and then reintroducing these modified cells—known as Chimeric Antigen Receptor T cells (CAR-T)—back into the patient to attack cancer cells. This treatment has shown high efficacy in certain types of blood cancer.
Cancer vaccines are another form of immunotherapy designed to stimulate the immune system's response to specific cancers. These vaccines typically contain specific antigens from cancer cells, which can activate the immune system to recognize and attack cells containing these antigens. Cancer vaccines can be preventative, aimed at preventing cancer from developing, or therapeutic, intended to treat existing cancer.
Immunomodulators are drugs that adjust the activity of the immune system, enhancing or modulating the immune response through various mechanisms. For example, some immunomodulators can enhance the ability of immune cells to attack cancer cells or alter the immune environment to be more conducive to immune system activity.
The research and application of cancer immunotherapy is a rapidly evolving field. As our understanding of the immune system and cancer biology deepens, new treatment methods continue to be developed. These therapies offer a different approach from traditional surgery, radiation, and chemotherapy, providing hope for some hard-to-treat cancers.
Despite the success of cancer immunotherapy in many cases, it also faces challenges. Not all patients respond to this treatment, and sometimes immunotherapy can trigger severe immune-related side effects. Therefore, scientists and doctors are striving to better understand which patients are most likely to benefit from immunotherapy and how to manage potential side effects during treatment. Additionally, researchers are exploring how to combine immunotherapy with other forms of cancer treatment to enhance treatment outcomes.
The development of immunotherapy still focuses on targeting specific points, thus facing similar side effects and resistance issues as targeted drugs. However, its advantage lies in its mechanism, which aligns with the long-term goals of cancer treatment: by activating the immune system and combining it with other therapies, a comprehensive effect can be achieved. The following table provides a detailed assessment of five key factors of immunotherapy.
7. Comprehensive Comparison
Using the same approach, we can apply the five-factor analysis framework to any cancer treatment. Here, we compared six common cancer treatment methods. According to the scoring results, tumor ablation emerged as the most effective, suggesting it should be the preferred method if patient-specific conditions are not considered. In contrast, chemotherapy received the lowest overall score, indicating that patients might consider avoiding it. However, the potential of combination therapies should not be overlooked. For example, combining tumor ablation with chemotherapy, using methods like intra-tumor injections of chemotherapy drugs similar to those used at the "Baofa" Hospital in China, might be considered off-label in the current medical system but could offer superior treatment outcomes.
In reality, cancer patients often undergo various treatment methods, making combination therapy a recommended approach. This method is similar to evaluating single therapies, and our analysis framework is equally applicable to assessing combination therapies composed of multiple treatments. While this adds complexity to the evaluation process, it holds significant value for patients.
Through comprehensive assessment tables, we can propose and evaluate various potential combination therapies, selecting the optimal approach based on scores, even if the therapy does not currently exist. For instance, combining surgery with immunotherapy may be more effective than combining tumor ablation with immunotherapy. By refining specific therapies within a reasonable framework, such as not completely excising tumors during surgery but primarily disrupting tumor vasculature and directly injecting immunotherapy drugs into the tumor, we may not only reduce surgical complexity but also release antigens and stimulate immune responses in the tumor microenvironment, potentially achieving better treatment outcomes.
Furthermore, we can further adjust existing combination therapies using first principles, such as exploring the possibility of delivering immunotherapy drugs directly during tumor ablation, an adjustment to the combination of tumor ablation and immunotherapy, similar to the treatment approaches focused on by the Williams Cancer Institute in the United States.
Therefore, if cancer patients understand the application of first principles in cancer treatment, they can evaluate both single therapies and combination therapies through the same process. They can not only assess these therapies but also make reasonable adjustments within the existing healthcare system. This deepens patients' understanding of their cancer, alleviates psychological burdens, and contributes to effective cancer treatment and the goal of achieving long-term high-quality life.
Why is Intratumoral Injection of CD the Most Effective Method?
https://clo2xuewuliu.substack.com/p/discussion-with-two-american-oncologists
On August 21, I injected 0.5ml of high-concentration chlorine dioxide (around 10,000 ppm) into the muscle near my left shoulder joint, where there was inflammation and noticeable pain. The injection was quite painful, and no anesthetic was used. The pain lasted for about 24 hours and then disappeared. The arthritis symptoms completely vanished.









Well done, Xuewu to describe the various treatments, much needed.
Keep up your good work for the Good Universal Antidote.